Neurologist 1: “We generally do not use microelectrode recordings for target location in the subthalamic nucleus. We use the patient’s clinical response to DBS test stimulation and adjust the location of the lead if we need to. However, our last patient had a remarkable microsubthalamotomy effect so we could only test whether the patient would have adverse effects. The patient should do OK, right?”
Neurologist 2: “Maybe.”
Neurologist 1: “Gosh, I hope we do not have to surgically revise the DBS lead.”
Neurologist 2: “Maybe you should have asked your question before doing the surgery?”
No one wants to have to revise a DBS lead placement because of a poor placement that fails to deliver efficacy. The efforts to be extended in preventing the necessity of DBS lead revision surgery are proportional to the risks of the revision surgery. Certainly, there is the 2% risk of significant perioperative morbidity and the approximate 0.1% risk of perioperative mortality with the initial surgery. However, this analysis underestimates the real risks. For example, even if prior to the original surgery there is a 10% risk of needing a second surgery to revise the DBS lead placement, the risk going into the first DBS lead placement surgery is 2% for the first surgery, plus a 0.2% risk related to the probability of needing a second surgery. The significance of the increased prior risk is proportional to the risk of requiring a second surgery. The risks described here are rough estimates because of the lack of publically available data. Also, the 10% risk for DBS lead revision surgery is probably an over estimation; however, this author is aware of an institution that had an approximate 9% revision rate.
Whether the rough risk analysis described above is sufficient to warrant a re-evaluation of DBS lead implantation methodology, is a question that should be addressed. Further, patients and their family members, caregivers, and friends expect that their physicians and surgeons will consider the question.
In the hypothetical case described above, at least, there was the absence of adverse effects with DBS lead test stimulation that should provide reasonable reassurance that adverse effects will not limit the post-operative treatment. Based on what is known about how the brain responds to DBS, there is no reason to believe that a microsubthalamotomy would disguise the risk for acute post-operative adverse effects, such as paresthesias, tonic muscle contraction, diplopia, and speech and language effects. The other half of the equation is the estimation of efficacy.
While there is little published on a systematic prospective study of the question whether a microsubthalamotomy is predictive of efficacy, Maltête et al. (Maltête D, Derrey S, Chastan N, Debono B, Gérardin E, Fréger P, Mihout B, Menard JF, Hannequin D. Microsubthalamotomy: an immediate predictor of long-term subthalamic stimulation efficacy in Parkinson disease. Mov Disord. 2008 May 15;23(7):1047-50. doi: 10.1002/mds.22054. PubMed PMID: 18412281) did a great service by publishing their retrospective analysis of 30 patients. The study was well done with the exception of the statistical analyses and the conclusions drawn. Thirty consecutive patients were studied as a way of avoiding sampling bias. The microsubthalamotomy and post-operative evaluations off drug and on stimulation were done carefully. In particular, the post-operative assessments were performed after 6 months, which is a reasonably sufficient amount of time to optimize the DBS electrode configurations and stimulation parameters.
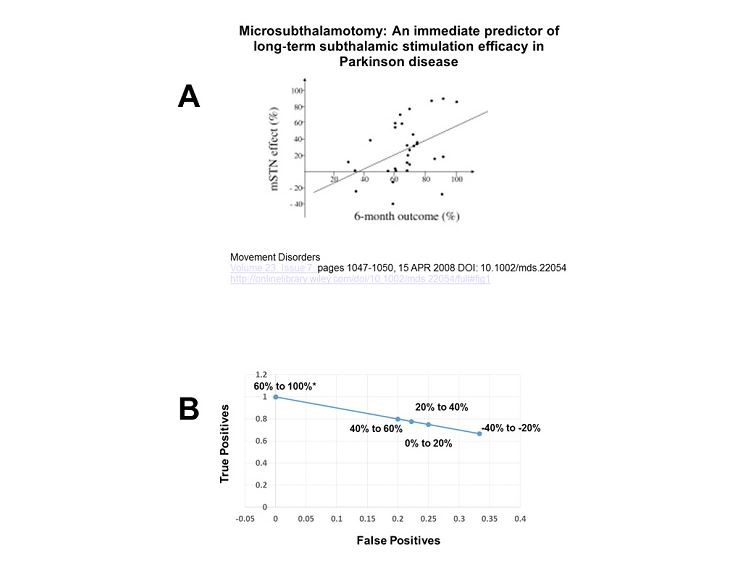
Unfortunately, the main conclusion relative to the predictive value of a microsubthalamotomy for post-operative outcome was based on a regression analysis. This is not optimal for assessing the diagnostic utility of a test. In this case, the degree of a microsubthalamotomy effect should be considered as a diagnostic test for optimal post-operative response. The problem is that a correlation may be statistically significant but of little diagnostic value. In the correlation published between the microsubthalamotomy score and the 6-month off meds – on DBS score, the correlation was significant at p = 0.005, leaving little doubt about a relationship (see figure). However, the correlation coefficient was 0.4, which roughly translates to an adjusted R2 of 0.13. Thus while there is a statistical correlation between the microsubthalamotomy and the 6-month off meds – on stimulation scores, the microsubthalamotomy score can only account for 13% of the variability in the post-operative score. Hardly a strong predictor.
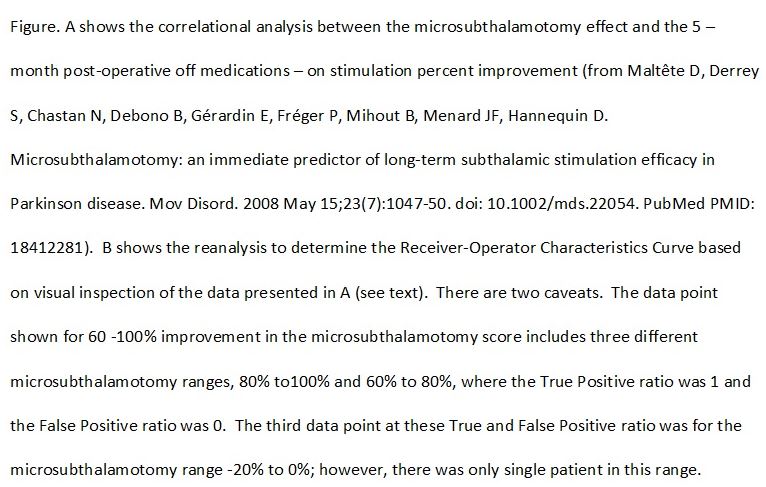
The more appropriate analysis would be to examine the Receiver-Operator Characteristics Curve that relates true and false positives. Reanalysis of the data provided by Maltête et al. is shown below. For the purposes of the reanalysis a positive result is a post-operative improvement of at least 50%. This is consistent with large scale prospective clinical trials. The finding of a positive result for any specific range of microsubthalamotomy scores is taken as a true positive, while the failure to achieve at least 50% improvement in the post-operative scores is taken as a false positive. Visual inspection of figure 1 in the study by Maltête et al. allowed determination of the true positive and false positive ratio for each 20% range in the microsubthalamotomy score. For example, of the 9 patients who had a microsubthalamotomy score between 0% – 20%, 7 had at least 50% improvement in post-operative score (taken as true positive score) while 2 had less than a 50% improvement (taken as a false positive). The Receiver-Operator Curve was plotted (see figure). As can be seen, the Receiver-Operator Curve is a flat line with a regression equation of y = -x +1. The area under a curve with this repression equation would be 0.5. This means, as a diagnostic test, the using the microsubthalamotomy score as a predictor of post-operative improvement would be operating at chance. In other words, one might just as well roll the dice. This is not unexpected, as the estimated adjusted R2 was so low. Also, the majority of patients who acutely worsened with the microsubthalamotomy had better than a 50% improvement post-operatively.
A caution needs to be taken about the analyses as the number of subjects was relatively small given the variability in the data. This was particularly important in determining the true and false positive rates for patients with a microsubthalamotomy score between -20% to 0%, where there was only a single subject.
Maltête et al. offered another important observation. Based on visual inspection of figure 1 in their paper (see figure below), 19 of the 30 patients (63%) had a microsubthalamotomy effect greater than 20% (positive or negative) relative to the pre-operative assessment. This means that without some other intraoperative method to validate the optimal location for the DBS lead, such as microelectrode recordings, the surgeon will only have neuroimaging studies, either pre- or intra-operatively. The majority of the time DBS lead testing for efficacy will likely be invalidated by the microsubthalamotomy effect.
The risk of a microsubthalamotomy effect compromising intra-operative DBS lead testing is directly proportional to the frequency of a microsubthalamotomy effect. The question is why there is such a high rate of microsubthalamotomy effects in the study by Maltête et al. This incidence seems higher than what this author would expect, based on the author’s anecdotal experience, but Maltête et al. did a careful study and their observations likely are valid, notwithstanding this author’s experience. The only possible explanation available to this author, and note it is a possibility not a probability, is the use of the five microelectrode array. In this author’s experience and reasoning, the use of five electrodes inserted simultaneously is more likely to traumatize local neural tissue and thereby increase the incidence of microsubthalamotomy effects (see GNC Professional Newsletter Microelectrode Arrays versus Single Electrodes).